ACCORDING TO THE FOLLOWING STANDARDS:
ISO 594-1
ISO 594-2
ISO 7886-1
ISO 7886-2 (COMPATIBLE WITH PUMP’S SETTING B.BRAUN OMNIFIX)
ISO 10993
UNI EN ISO 11135
UNI EN ISO 556
EUROPEAN PHARMACOPOEIAE
DIRETTIVA EUROPEA 93/42 CEE
I DISPOSITIVI MEDICI E I DOCUMENTI ASSOCIATI SONO RISERVATI ESCLUSIVAMENTE AD OPERATORI SANITARI
M series
Opaque syringe for infusion systems with needles
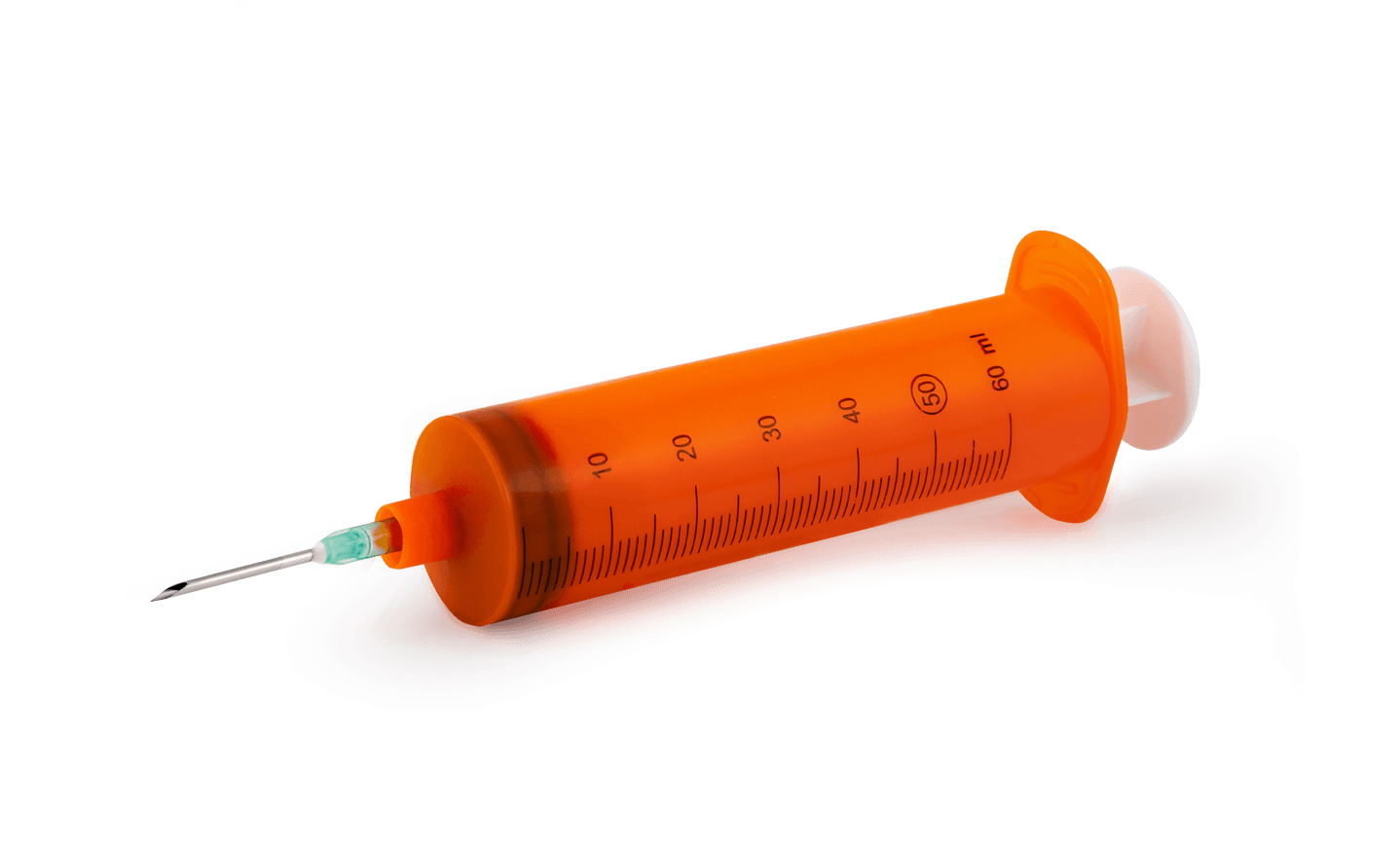
ML99NASingle-use 50/60ml Luer lock cone syringe
Pump AdjustmentCompatibile Braun Omnifix 50*
Box/Pcs50 (UDI-DI 08033481792258)
Carton/Pcs300 (UDI-DI 08033481792265)
*There is no business agreement or any other type of agreement between I.M.I. Snc and B.Braun Melsungen AG. The B.Braun Omnifix brand is only used for descriptive purpose to ensure correct use of the device.

Technical datas
N° IDENTIFICATION CE:0373
NUMBER OF THE CERTIFICATE: QPZ-1931-20
CLASS:IIa
CND / EMDN CLASSIFICATION:A020102020101
N° REGISTRATION REPERTORY D.M. :59510 (Italian registration)
LABEL:IMI
VOLUME:50/60ML
NOZZLE:LUER LOCK
MOUNTED NEEDLE14G
BARREL:ATOXIC POLYPROPYLENE ADDED OF MASTER BATCH ORANGE COLOR
PLUNGER:POLYPROPYLENE ATOXIC ADDED OF MASTER BATCH WHITE COLOR
SEAL GASKET DOUBLE RING:MADE OF SYNTHETIC POLYSOPRENE BLACK COLOR
SCALE:MARKED EACH ML IN BLUE COLOR FOR DRYING PROCESS BY UV LED
SILICONIC OIL: MEDICAL FLUID
PRIMARY PACKAGE:BLISTER OF MEDICAL PAPER AND COUPLED FILM
SECONDARY PACKAGE:BOX OF TRIPLE CARTON RECYCLABLE x 50 PCS cm 38.0x24.5x19.5
OUTSIDE CARTON:TRIPLE CARTON RECYCLABLE x 300 PCS cm 60.0x39.0x49.5
STERILIZATION:BY ETHYLENE OXYDE
SHELF LIFE:5 YEARS
DELIVERY:ON PALLETT 120x80x210 INCLUDING 16 OUTSIDE CARTONS
MANUFACTURE:ACCORDING TO A QUALITY SYSTEM THAT ACCOMPLISHES ANNEX V° OF DIRECTIVE 93/42/CEE
NOTE: SYRINGES ARE PVC FREE, LATEX FREE. ALL USED SUBSTANCES ACCOMPLISH WITH REGULATION EC 1907/2006 REACH

