ACCORDING TO THE FOLLOWING STANDARDS:
ISO 594-1
ISO 594-2
ISO 7886-1
ISO 10993
UNI EN ISO 11135
UNI EN ISO 556
EUROPEAN PHARMACOPOEIAE
EUROPEAN DIRECTIVE 93/42 CEE
I DISPOSITIVI MEDICI E I DOCUMENTI ASSOCIATI SONO RISERVATI ESCLUSIVAMENTE AD OPERATORI SANITARI
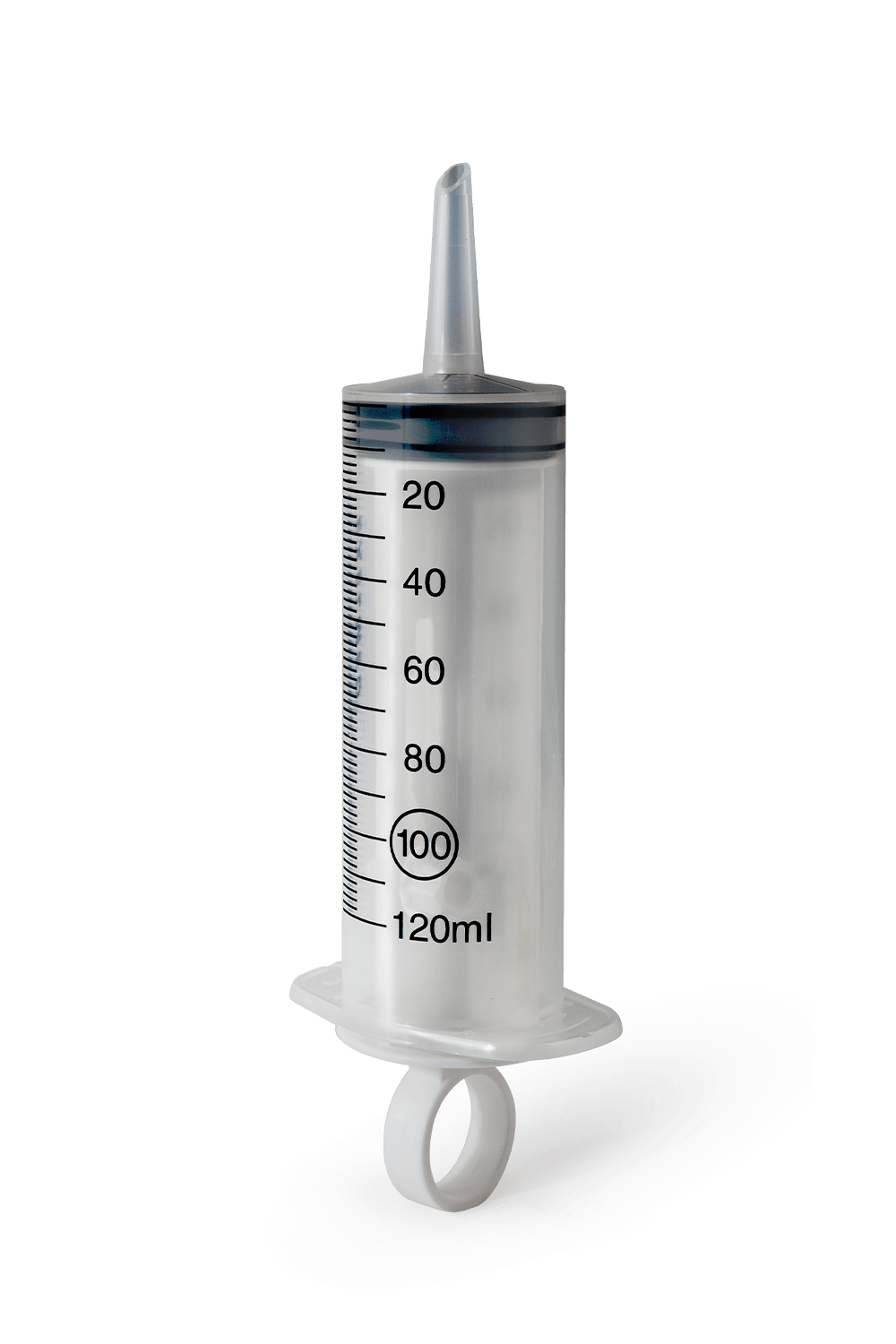
Transparent syringe with eccentric Luer cone.
TC98NSingle-use syringe 100/120ml catheter cone
Box/Pcs30 (UDI-DI 08033481793118)
Carton/Pcs180 (UDI-DI 08033481793125)
Technical datas
N° IDENTIFICATION CE:0373
NUMBER OF THE CERTIFICATE: QPS-0530-20
CLASS:Is
CND / EMDN CLASSIFICATION:A02010203
N° REGISTRATION REPERTORY D.M. :58580
LABEL:IMI
VOLUME:100/120ML
NOZZLE:CATHETER
BARREL:TRANSPARENT POLYPROPYLENE ATOXIC
PLUNGER:POLYPROPYLENE ATOXIC ADDED OF MASTER BATCH WHITE COLOR
N° 2 LUER ADAPTERS
SEAL GASKET DOUBLE RING:MADE OF TPE BLACK COLOR
SCALE:MARKED EACH 2 ML IN BLACK COLOR THERMOPRINTED
SILICONIC OIL: MEDICAL FLUID
PRIMARY PACKAGE:BLISTER OF MEDICAL PAPER AND COUPLED FILM
SECONDARY PACKAGE:BOX OF TRIPLE CARTON RECYCLABLE x 30 PCS cm 38.0x24.5x19.5
OUTSIDE CARTON:TRIPLE CARTON RECYCLABLE x 180 PCS cm 60.0x39.0x49.5
STERILIZATION:BY ETHYLENE OXYDE
SHELF LIFE:5 YEARS
DELIVERY:ON PALLETT 120x80x210 INCLUDING 16 OUTSIDE CARTONS
MANUFACTURE:ACCORDING TO A QUALITY SYSTEM THAT ACCOMPLISHES ANNEX V° OF DIRECTIVE 93/42/CEE
NOTE:SYRINGES ARE PVC FREE, LATEX FREE. ALL USED SUBSTANCES ACCOMPLISH WITH REGULATION EC 1907/2006 REACH.

