ACCORDING TO THE FOLLOWING STANDARDS:
ISO 594-1
ISO 594-2
ISO 7886-1
ISO 10993
UNI EN ISO 11135
UNI EN ISO 556
EUROPEAN PHARMACOPOEIAE
EUROPEAN DIRECTIVE 93/42 CEE
I DISPOSITIVI MEDICI E I DOCUMENTI ASSOCIATI SONO RISERVATI ESCLUSIVAMENTE AD OPERATORI SANITARI
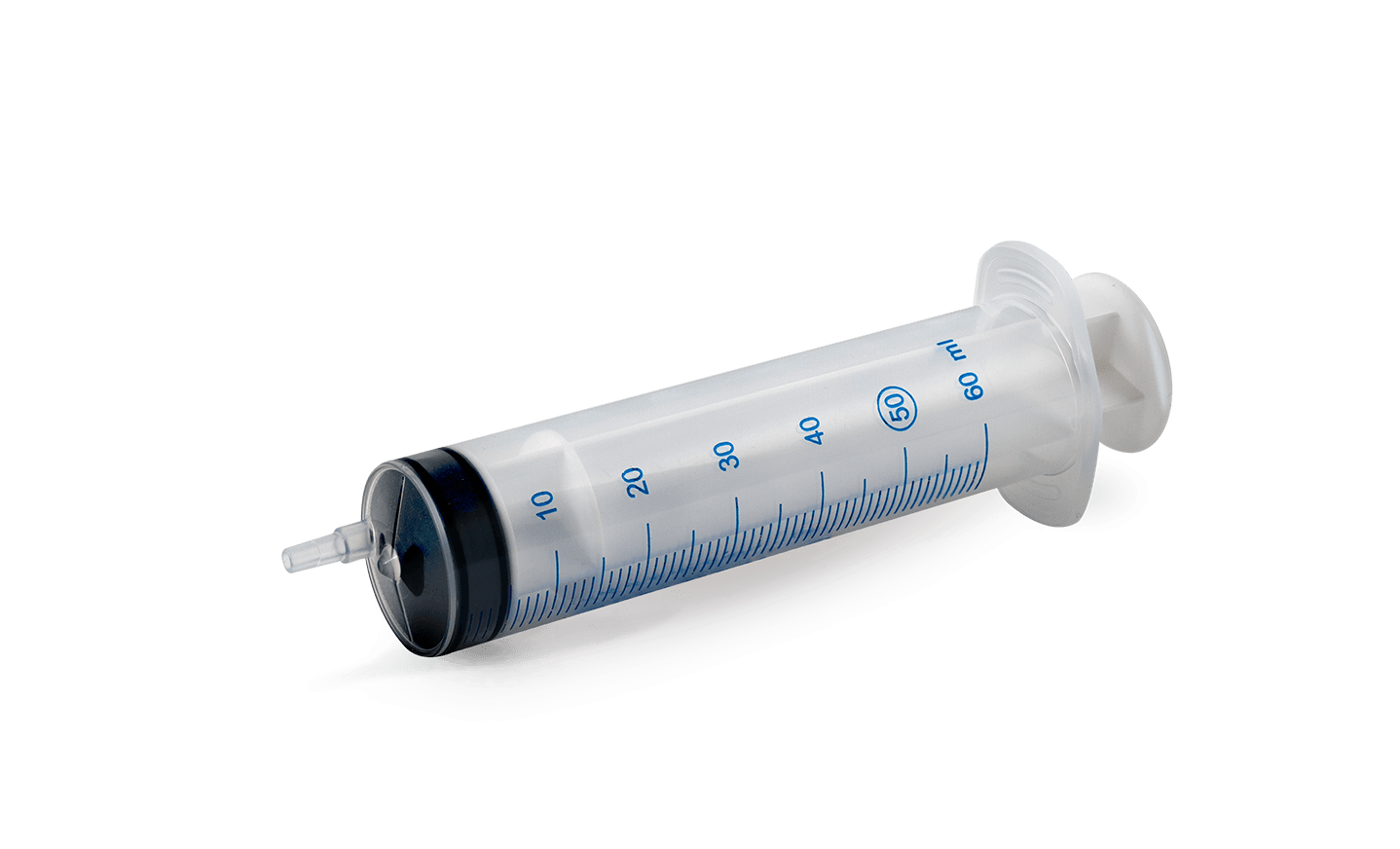
Transparent syringe for infusion systems
TE99NSingle-use syringe 50/60ml Luer eccentric nozzle
Box/Pcs50 (UDI-DI 08033481791916)
Carton/Pcs300 (UDI-DI 08033481791923)

Technical datas
N° IDENTIFICATION CE:0373
NUMBER OF THE CERTIFICATE: QPS-0530-20
CLASS:Is
CND / EMDN CLASSIFICATION:A020102020102
N° REGISTRATION REPERTORY D.M. :59480 (Italian registration)
LABEL:IMI
VOLUME:50/60ML
NOZZLE:LUER ECCENTRIC
BARREL:TRANSPARENT POLYPROPYLENE ATOXIC
PLUNGER:POLYPROPYLENE ATOXIC ADDED OF MASTER BATCH WHITE COLOR
SEAL GASKET DOUBLE RING:MADE OF TPE BLACK COLOR
SCALE:MARKED EACH ML IN BLU COLOR FOR DRYING PROCESS BY UV LED
SILICONIC OIL:MEDICAL FLUID
PRIMARY PACKAGE:BLISTER OF MEDICAL PAPER AND COUPLED FILM
SECONDARY PACKAGE:BOX OF TRIPLE CARTON RECYCLABLE x 50 PCS cm 38.0x19.5x19.5
OUTSIDE CARTON:TRIPLE CARTON RECYCLABLE x 300 PCS cm 60.0x39.0x42.0
STERILIZATION:BY ETHYLENE OXYDE
SHELF LIFE:5 YEARS
DELIVERY:ON PALLETT 120x80x220 INCLUDING 20 OUTSIDE CARTONS
MANUFACTURE:ACCORDING TO A QUALITY SYSTEM THAT ACCOMPLISHES ANNEX V° OF DIRECTIVE 93/42/CEE
NOTE: SYRINGES ARE PVC FREE, LATEX FREE. ALL USED SUBSTANCES ACCOMPLISH WITH REGULATION EC 1907/2006 REACH.

